3. 结构
3.1 二维结构
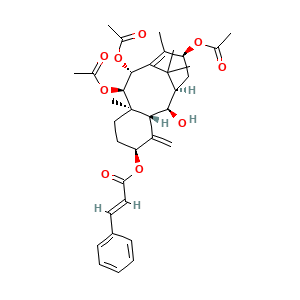
3.2 三维结构
-1
-2
-3
88 91 0 1 0 0 0 0 0999 V2000
2.2074 -3.6322 1.2897 O 0 0 0 0 0 0 0 0 0 0 0 0
3.0395 1.0017 -2.3536 O 0 0 0 0 0 0 0 0 0 0 0 0
2.9884 2.3593 0.0548 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.4934 -0.9301 1.9068 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.5689 -2.2826 -1.0644 O 0 0 0 0 0 0 0 0 0 0 0 0
4.9298 2.1436 -1.6761 O 0 0 0 0 0 0 0 0 0 0 0 0
1.3635 3.5206 1.2159 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.0165 -1.8651 3.9535 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.1798 -2.3661 -2.7185 O 0 0 0 0 0 0 0 0 0 0 0 0
1.9280 -1.0372 -1.5619 C 0 0 2 0 0 0 0 0 0 0 0 0
1.3604 -1.9858 -0.3970 C 0 0 2 0 0 0 0 0 0 0 0 0
2.1136 -1.4170 2.1513 C 0 0 2 0 0 0 0 0 0 0 0 0
2.5679 0.0596 2.0613 C 0 0 0 0 0 0 0 0 0 0 0 0
2.3104 -2.2756 0.8300 C 0 0 1 0 0 0 0 0 0 0 0 0
2.7146 0.2712 -1.1466 C 0 0 2 0 0 0 0 0 0 0 0 0
1.5709 0.7291 1.0991 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7135 -0.6194 -2.4655 C 0 0 0 0 0 0 0 0 0 0 0 0
2.0569 1.3050 -0.2055 C 0 0 1 0 0 0 0 0 0 0 0 0
0.6769 -1.5055 2.7441 C 0 0 0 0 0 0 0 0 0 0 0 0
0.6714 -3.1800 -1.0975 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.1492 -1.7776 -2.9893 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.2808 -0.3422 2.3969 C 0 0 1 0 0 0 0 0 0 0 0 0
2.9371 -1.8485 -2.4404 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.5443 -2.7919 -1.9138 C 0 0 2 0 0 0 0 0 0 0 0 0
0.2410 0.6531 1.3736 C 0 0 0 0 0 0 0 0 0 0 0 0
4.0918 0.1227 1.7716 C 0 0 0 0 0 0 0 0 0 0 0 0
2.4749 0.7812 3.4461 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.8268 1.4556 0.6722 C 0 0 0 0 0 0 0 0 0 0 0 0
1.1072 -4.4504 -1.1575 C 0 0 0 0 0 0 0 0 0 0 0 0
3.8081 2.1029 -2.1632 C 0 0 0 0 0 0 0 0 0 0 0 0
2.5009 3.4065 0.7792 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.0726 -1.8475 2.7314 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.7177 -1.8985 -1.6898 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0614 3.3067 -2.6525 C 0 0 0 0 0 0 0 0 0 0 0 0
3.5807 4.4276 0.9734 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.8099 -2.8616 1.9103 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.3188 -0.8004 -0.8993 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.0582 0.1309 -1.5182 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.7154 1.2632 -0.8418 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.9446 1.1977 0.5231 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.0909 2.3716 -1.5834 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.5662 2.2696 1.1637 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.7123 3.4436 -0.9428 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.9500 3.3925 0.4307 C 0 0 0 0 0 0 0 0 0 0 0 0
0.5140 -1.4554 0.0372 H 0 0 0 0 0 0 0 0 0 0 0 0
2.7554 -1.9009 2.9048 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3412 -2.1859 0.4806 H 0 0 0 0 0 0 0 0 0 0 0 0
3.6826 -0.0408 -0.7605 H 0 0 0 0 0 0 0 0 0 0 0 0
0.0605 0.0514 -1.8962 H 0 0 0 0 0 0 0 0 0 0 0 0
1.0579 -0.0451 -3.3341 H 0 0 0 0 0 0 0 0 0 0 0 0
1.2420 1.7543 -0.7808 H 0 0 0 0 0 0 0 0 0 0 0 0
0.2647 -2.4875 2.4858 H 0 0 0 0 0 0 0 0 0 0 0 0
0.7762 -1.5226 3.8391 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.0175 -1.3475 -3.4985 H 0 0 0 0 0 0 0 0 0 0 0 0
0.4189 -2.3008 -3.7696 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.5445 0.2192 3.3027 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3144 -1.2509 -3.2778 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5033 -2.7449 -2.8896 H 0 0 0 0 0 0 0 0 0 0 0 0
3.8016 -2.1659 -1.8472 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.9550 -3.6790 -2.4150 H 0 0 0 0 0 0 0 0 0 0 0 0
4.4401 1.1461 1.6050 H 0 0 0 0 0 0 0 0 0 0 0 0
4.4270 -0.4903 0.9393 H 0 0 0 0 0 0 0 0 0 0 0 0
4.6567 -0.2574 2.6336 H 0 0 0 0 0 0 0 0 0 0 0 0
3.0412 0.2338 4.2095 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4630 0.8990 3.8369 H 0 0 0 0 0 0 0 0 0 0 0 0
2.8973 1.7920 3.3842 H 0 0 0 0 0 0 0 0 0 0 0 0
2.9521 -3.8008 1.8917 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.4680 2.3689 0.1970 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.5930 1.7709 1.3892 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3007 0.8514 -0.1014 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0316 -4.7804 -0.7026 H 0 0 0 0 0 0 0 0 0 0 0 0
0.5659 -5.2032 -1.7225 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0269 3.3123 -2.3032 H 0 0 0 0 0 0 0 0 0 0 0 0
3.0804 3.3197 -3.7455 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5510 4.2121 -2.2843 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7966 4.9282 0.0277 H 0 0 0 0 0 0 0 0 0 0 0 0
4.4801 3.9482 1.3690 H 0 0 0 0 0 0 0 0 0 0 0 0
3.2457 5.1726 1.7009 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1099 -3.6867 2.5647 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.1549 -3.2817 1.1466 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.7141 -2.4270 1.4835 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.0982 -0.7554 0.1516 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.2125 0.0737 -2.5936 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6816 0.3365 1.1292 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.9111 2.4268 -2.6537 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.7555 2.2285 2.2324 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.0111 4.3182 -1.5133 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.4348 4.2267 0.9292 H 0 0 0 0 0 0 0 0 0 0 0 0
1 14 1 0 0 0 0
1 67 1 0 0 0 0
2 15 1 0 0 0 0
2 30 1 0 0 0 0
3 18 1 0 0 0 0
3 31 1 0 0 0 0
4 22 1 0 0 0 0
4 32 1 0 0 0 0
5 24 1 0 0 0 0
5 33 1 0 0 0 0
6 30 2 0 0 0 0
7 31 2 0 0 0 0
8 32 2 0 0 0 0
9 33 2 0 0 0 0
10 11 1 0 0 0 0
10 15 1 0 0 0 0
10 17 1 0 0 0 0
10 23 1 0 0 0 0
11 14 1 0 0 0 0
11 20 1 0 0 0 0
11 45 1 0 0 0 0
12 13 1 0 0 0 0
12 14 1 0 0 0 0
12 19 1 0 0 0 0
12 46 1 0 0 0 0
13 16 1 0 0 0 0
13 26 1 0 0 0 0
13 27 1 0 0 0 0
14 47 1 0 0 0 0
15 18 1 0 0 0 0
15 48 1 0 0 0 0
16 18 1 0 0 0 0
16 25 2 0 0 0 0
17 21 1 0 0 0 0
17 49 1 0 0 0 0
17 50 1 0 0 0 0
18 51 1 0 0 0 0
19 22 1 0 0 0 0
19 52 1 0 0 0 0
19 53 1 0 0 0 0
20 24 1 0 0 0 0
20 29 2 0 0 0 0
21 24 1 0 0 0 0
21 54 1 0 0 0 0
21 55 1 0 0 0 0
22 25 1 0 0 0 0
22 56 1 0 0 0 0
23 57 1 0 0 0 0
23 58 1 0 0 0 0
23 59 1 0 0 0 0
24 60 1 0 0 0 0
25 28 1 0 0 0 0
26 61 1 0 0 0 0
26 62 1 0 0 0 0
26 63 1 0 0 0 0
27 64 1 0 0 0 0
27 65 1 0 0 0 0
27 66 1 0 0 0 0
28 68 1 0 0 0 0
28 69 1 0 0 0 0
28 70 1 0 0 0 0
29 71 1 0 0 0 0
29 72 1 0 0 0 0
30 34 1 0 0 0 0
31 35 1 0 0 0 0
32 36 1 0 0 0 0
33 37 1 0 0 0 0
34 73 1 0 0 0 0
34 74 1 0 0 0 0
34 75 1 0 0 0 0
35 76 1 0 0 0 0
35 77 1 0 0 0 0
35 78 1 0 0 0 0
36 79 1 0 0 0 0
36 80 1 0 0 0 0
36 81 1 0 0 0 0
37 38 2 0 0 0 0
37 82 1 0 0 0 0
38 39 1 0 0 0 0
38 83 1 0 0 0 0
39 40 2 0 0 0 0
39 41 1 0 0 0 0
40 42 1 0 0 0 0
40 84 1 0 0 0 0
41 43 2 0 0 0 0
41 85 1 0 0 0 0
42 44 2 0 0 0 0
42 86 1 0 0 0 0
43 44 1 0 0 0 0
43 87 1 0 0 0 0
44 88 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
[(1R,2R,3R,5S,8R,9R,10R,13S)-9,10,13-triacetyloxy-2-hydroxy-8,12,15,15-tetramethyl-4-methylidene-5-tricyclo[9.3.1.03,8]pentadec-11-enyl] (E)-3-phenylprop-2-enoate
4.2 InChl
InChI=1S/C35H44O9/c1-19-26(44-28(39)15-14-24-12-10-9-11-13-24)16-17-35(8)29(19)31(40)25-18-27(41-21(3)36)20(2)30(34(25,6)7)32(42-22(4)37)33(35)43-23(5)38/h9-15,25-27,29,31-33,40H,1,16-18H2,2-8H3/b15-14+/t25-,26-,27-,29-,31+,32+,33-,35+/m0/s1
4.3 InChlKey
NQINDQGQJLIYFL-PBRGCZQGSA-N
4.4 Canonical SMILES
CC1=C2C(C(C3(CCC(C(=C)C3C(C(C2(C)C)CC1OC(=O)C)O)OC(=O)C=CC4=CC=CC=C4)C)OC(=O)C)OC(=O)C
4.5 lsomeric SMILES
CC1=C2[C@H]([C@@H]([C@@]3(CC[C@@H](C(=C)[C@H]3[C@@H]([C@@H](C2(C)C)C[C@@H]1OC(=O)C)O)OC(=O)/C=C/C4=CC=CC=C4)C)OC(=O)C)OC(=O)C
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病